A guanidine CNPq moiety has never been addressed as a catalytic group in families of glycosyl hydrolases Nevertheless, an Arg residue is present in the active site region of S frugi perda family 1 bglycosidase that is responsible for the References stabilization of the charged nucleophile (Marana et al, Behm, CA, 1997 The role of trehalose in the physiology of NemaThe guanidine group in the Q monomer, incorporated via a twocarbon linker, is predicted to form two hydrogen bonds with the G base in a CG pair (Figure 1D) (27, 28) Thus, the incorporation of Q residues into triplexforming PNAs may improve the binding affinity and sequence specificity to an RNA duplex with an inverted Watson–Crick CG pair (Figure 2B and G )Guanidinium is a guanidinium ion It is a conjugate acid of a guanidine and a carbamimidoylazanium A strong organic base existing primarily as guanidium ions at physiological pH It is found in the urine as a normal product of protein metabolism It is also used in
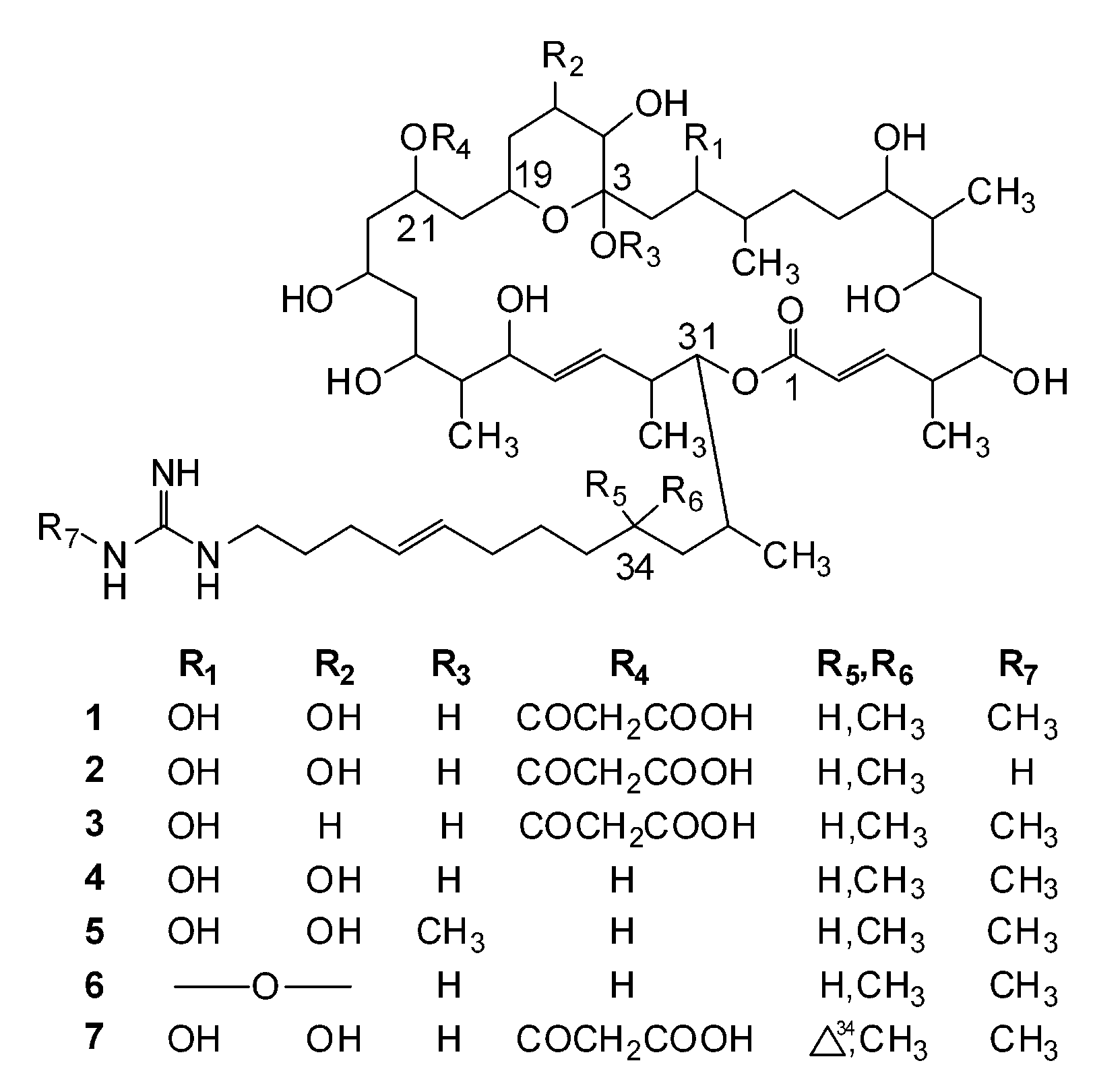
Molecules Free Full Text Guanidine Containing Polyhydroxyl Macrolides Chemistry Biology And Structure Activity Relationship Html
Guanidine chemical structure
Guanidine chemical structure-In the present study, the effects on the aggregation of hen eggwhite lysozyme via incubation in concentrated solutions of three different chaotropic agents namely guanidine thiocyanate, guanidine hydrochloride and urea were investigated Here we used three different methods for the detection of the aggregates, thioflavin T fluorescence, circular dichroism spectroscopy andD Crystal structure of the ligandbound guanidineI riboswitch aptamer d Guanidinium recognized via Hbonding, ionic interactions, and cationp interactions d Unpredicted P3 helix contains binding site and reveals possible switching mechanism Authors Caroline W Reiss, Yong Xiong, Scott A Strobel Correspondence scottstrobel@yaleedu In Brief Reiss et al present the 27 A˚ crystal



Recent Advances In Guanidine Based Organocatalysts In Stereoselective Organic Transformation Reactions Intechopen
The guanidine group is present in ntiviral, and as anticancer drugs The guanidine sidegroup contributes to theSodium hypobromite (NaOBr) PROCEDURE Take 1 ml of original solution (protein solution) in a test tube Add 1 ml of 5% sodium hydroxide to the test tube And add 2 drops of 1% alcoholic αnaphtholGuanidine Suppliers USA Find where to buy products from suppliers in the USA, including distributors, industrial manufacturers in America, bulk supplies and wholesalers of raw ingredients & finished goods Search for products or services, then visit the American suppliers website for prices, SDS or more information You can also view suppliers in Australia, NZ or the UK
The present result is in close agreement with the same carbon atom) functional group 10–12 Also, guanidine reported results 3 The grown guanidine carbonate doped NSH derivatives are versatile intermediates used in the manufacture crystal was subjected to powder Xray diffraction studies using a of plastics, resins, rubber chemicals, photo chemicals, fungicides, Rich Seifert XA) There is a binding region for the imidazole ring of histamine analogues which is common for agonists and antagonists b) There is a binding region which interacts ionically with the αnitrogen of histamine and results in agonist activity c) There is aThe guanidine group is a common key unit in various natural and synthetic compounds demonstrating antimicrobial, antiviral, and antitumor activities 26 High symmetry of the Yshaped guanidinium
The interaction of guanidinetype cationic surfactants with bovine serum albumin (BSA) and liposome were investigated Dodecylguanidine hydrochloride (C(12)A(0)G) and several dodecanoylamide alkylguanidine hydrochlorides (C(12)A(m)G, m = 2, 3, 4, 6) were used as the guanidinetype surfactants In th · As soon as an anion binds to guanidine (forming a guanidiniumanion salt), the absorption spectrum of the chromophore attached to the guanidine group shifts If this shift is selective enough, we may use this property to tell not only about anions present in the solution, but also about their natureCarbonate de guanidine de qualité industrielle Rapport d'étude et d'analyse du marché mondial Le rapport de recherche sur le marché des Carbonate de guanidine de qualité industrielle transmet une analyse complète de cet espace de marché tout en offrant des données analytiques relatives aux segments qui influencent l'âge du revenu tout comme le développement des affaires
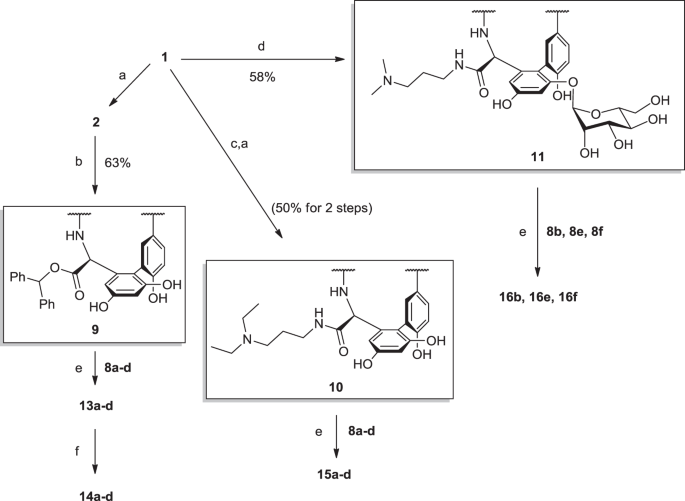


N Terminal Guanidine Derivatives Of Teicoplanin Antibiotics Strongly Active Against Glycopeptide Resistant Enterococcus Faecium The Journal Of Antibiotics
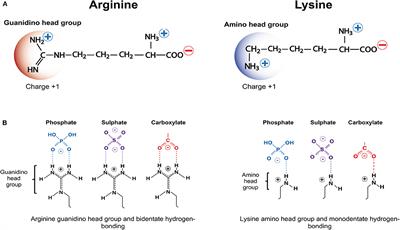


Frontiers Cationic Arginine Rich Peptides Carps A Novel Class Of Neuroprotective Agents With A Multimodal Mechanism Of Action Neurology
You could say theCertain Guanidine Derivatives 117 The three valences represented by the lines connecting a carbon atom with y, 8, and e may be satisfied by various atoms or groups of atoms, or all three may be taken up by a single nitrogen atom, in dicyandiamide In the present state of knowledge, a positive reaction with anything occurWith COVID19 ever present on our minds, viral research is being focused on now more than anything else We at AG Scientific have remained open to meet the needs of the research community and are stocking chemical agents such as guanidine thiocyanate Read on to learn more about this chemical and how it can aid your lab research
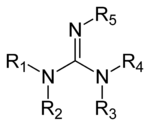


Guanidine Wikipedia
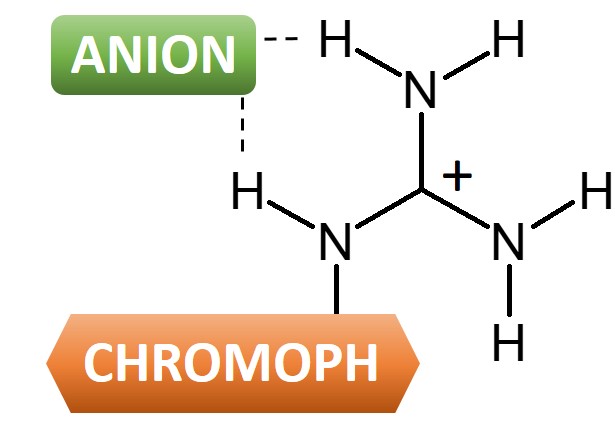


Guanidinium A New Analytical Tool To Detect Anions Light And Molecules
An unusual type of π‐electron delocalization in Y‐shaped molecules related to guanidine and its protonated form, the guanidinium ion, has been studiedDodine belongs to the M7 group of guanidine fungicides and is used for the control of pathogenic fungi affecting the foliage and fruit of certain crops hcscgcca Revendications pour l'Etat contractant suivant ES Procédé de préparation d'une composition pour lyser des cellules dans un échantillon comprenant la combinaison de 1 à 15 mM de Tris, de 0,1 à 2 mM d'EDTA, de 1 àThe guanidinium functional group is commonly used by proteins and enzymes to recognize and bind anions using ion pairing and hydrogen bonding The specific patterns of hydrogen bonding and the great basicity of the guanidine functional group allow it to play several key roles in recognition and catalysis This chapter outlines recent findings of guanidinium groups in natural systems and



Recent Advances In Guanidine Based Organocatalysts In Stereoselective Organic Transformation Reactions Intechopen


Introduction Of Anti Histamine Ppt Video Online Download
Guanidine containing peptides reported in the last three years 1 Introduction The guanidine motif is present in a great number of natural compounds that carry out different and important functions in animals and plants (Fig 1)1 Due to their appealing properties, many research'However, experience with guanidine and biguanides prompted the development of metformin' 'When guanidine hydrochloride is present these complexes dissociate into smaller units' 'Galega officinalis is rich in guanidine, the hypoglycemic component' 'RNA extraction was performed by the guanidine hydrochloride method'Here is presented some results obtained with a group of molecules composed of two antibiotics and 4 chemicals having in common the presence of two guanidine groups each possess an imine group and aminoacetal functional group These molecules when added in low concentrations interact rapidly with the infectious prion proteins (PrPsc) via hydrogen bond transfer between each of the guanidine



Glyoxal Wikipedia



Guanidino Group Is Present In The Amino Acid
Guanidine hydrochloride has also typically been used for the isolation of RNA, to denature globular proteins, and for protein refolding studies It can also be used to facilitate the generation of tryptic peptides for analysis of complex protein samples Physical form This product is a readytouse 6 M guanidine hydrochloride solution The 6 M Guanidine hydrochloride solution may be used as aGuanidine Hydrochloride Section 1 Identification 11 Product Identifiers Product Name if present and easy to do Continue rinsing P321 Specific treatment (see supplemental first aid instructions on this SDS) P330 Rinse mouth P332P313 If skin irritation occurs Get medical advice/ attention P337P313 If eye irritation persists Get medical advice/attention P362 TakeArginine test is specific for Arginine and indicates the presence of guanidine group in the arginine molecule Reagents Protein solution;



Antimicrobial Drugs Bearing Guanidine Moieties A Review Sciencedirect



Guanidine An Overview Sciencedirect Topics
· Despite the diverse functionalities present in the guanidine group, its synthesis for newer organocatalytic applications of guanidine is a relatively new research area in chiral compound synthesis Structurally, guanidine organocatalysts can be classified into several categories such as open chain 8 , monocyclic 9 , and bicyclic 10 structures as shown in Figure 4Guanine, an organic compound belonging to the purine group, a class of compounds with a characteristic tworinged structure, composed of carbon and nitrogen atoms, and occurring free or combined in such diverse natural sources as guano (the accumulated excrement and dead bodies of birds, bats, and seals), sugar beets, yeast,Guanidines are a group of organic compounds sharing a common functional group with the general structure (R 1 R 2 N)(R 3 R 4 N)C=N−R 5 The central bond within this group is that of an imine , and the group is related structurally to amidines and ureas



Antimicrobial Drugs Bearing Guanidine Moieties A Review Sciencedirect



Classical Guanidine Synthesis Guanidine Core Structure Obtained By Download Scientific Diagram
Time and NADPHdependent inhibition of cytochrome P450 3 by the cyclopentapeptide cilengitide significance of the guanidine group and accompanying spectral changes Bojić M, Barbero L, Dolgos H, Freisleben A, Gallemann D, Riva S, Guengerich FP Drug Metab Dispos 14 Sep;42(9) Epub 14 Jul 1 doi /dmd PMID SynthesisThis reaction will occur in the presence of the anaphthol catalyst and a guanidine group that is present in the peptone of the MRVP medium As a result, a pink complex is formed, imparting a rose color to the medium Development of a deep rose color in the culture 15 minutes following the addition of Barritt's reagent is indicative of the presence of acetyl methyl carbinol and representsThree binding regions were proposed to be present in the binding site of the H 2 receptor Which of the following statements is incorrect?
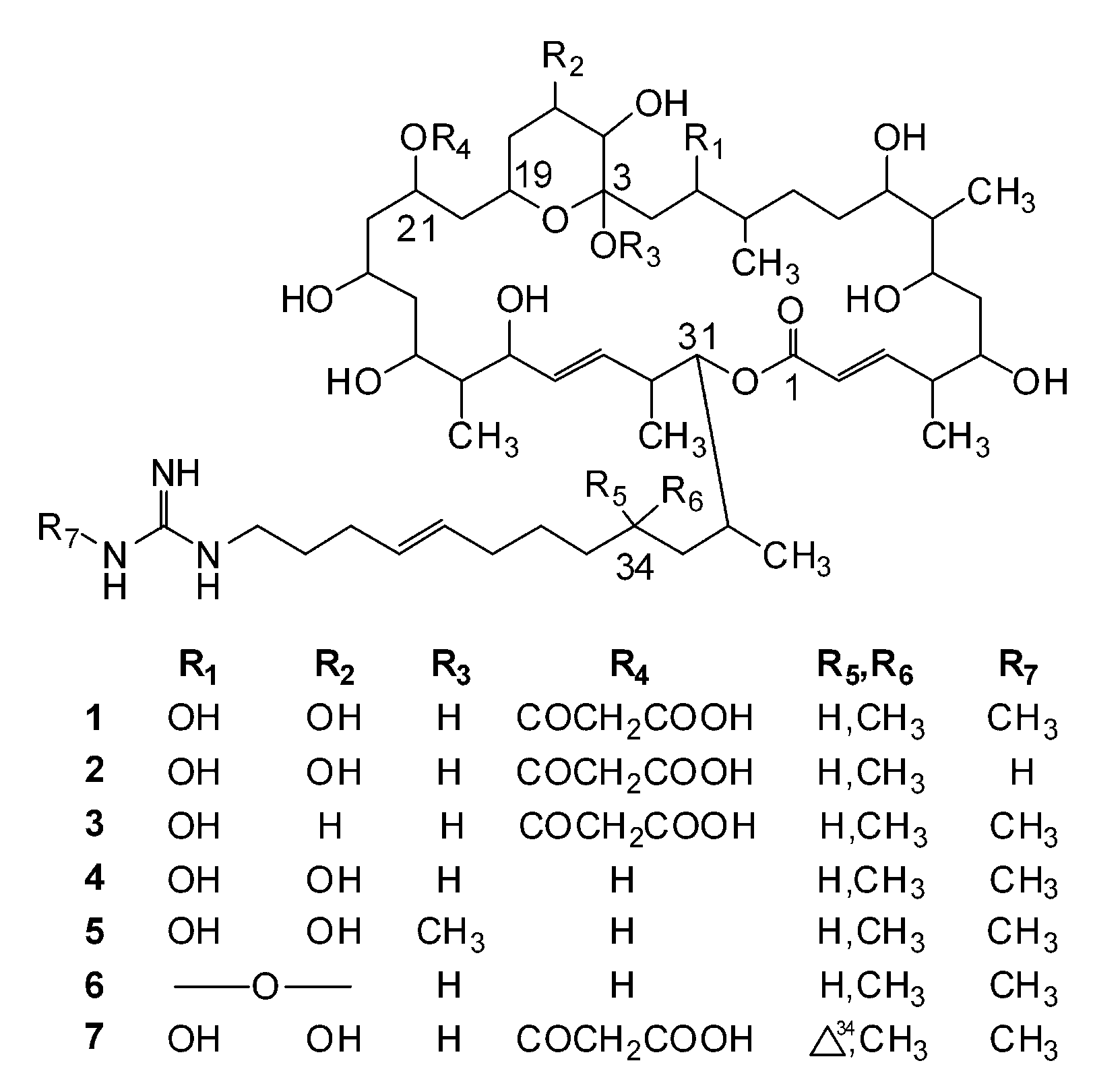


Molecules Free Full Text Guanidine Containing Polyhydroxyl Macrolides Chemistry Biology And Structure Activity Relationship Html
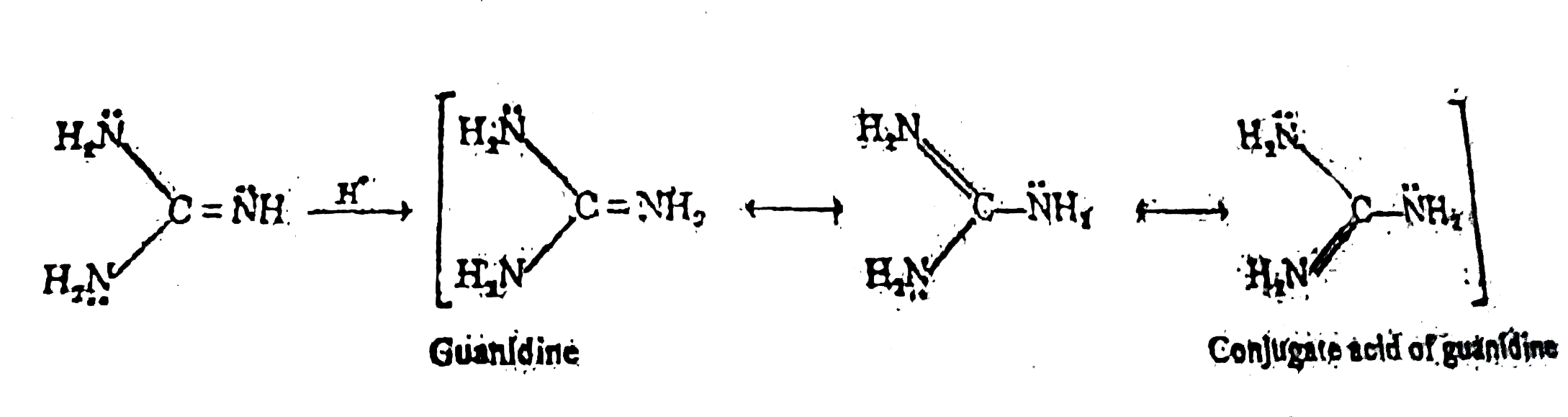


Guanidine Nh 2 2 C Nh Is Said To Be The Strongest Nitrogen Co
However, Nitrogen atoms are only Basic when they are part of a functional group such as an Amine or Guanidine The nitrogen lone pair is delocalized The reason that the Amide Nitrogen is no longer basic, as compared to an Amine, is that the lone pair of the Nitrogen is delocalized via resonance across the Nitrogen as well as the Carbon and Oxygen atoms of the carbonyl group (C=O) InHowever, guanidine has a pKa of 125(3) and should exist almost entirely as a cation under environmental conditions (pH 59)(SRC) As a result, guanidine may have greater adsorption and less mobility than its estimated Koc value indicates since cations generally adsorb more strongly to soils containing organic carbon and clay than neutral species(4) Volatilization from moist soilGuanidine moieties represented as hydrophilic functional groups are also present in the sidechain arginine amino acid, which has been observed in various enzyme active sites and motifs of cell
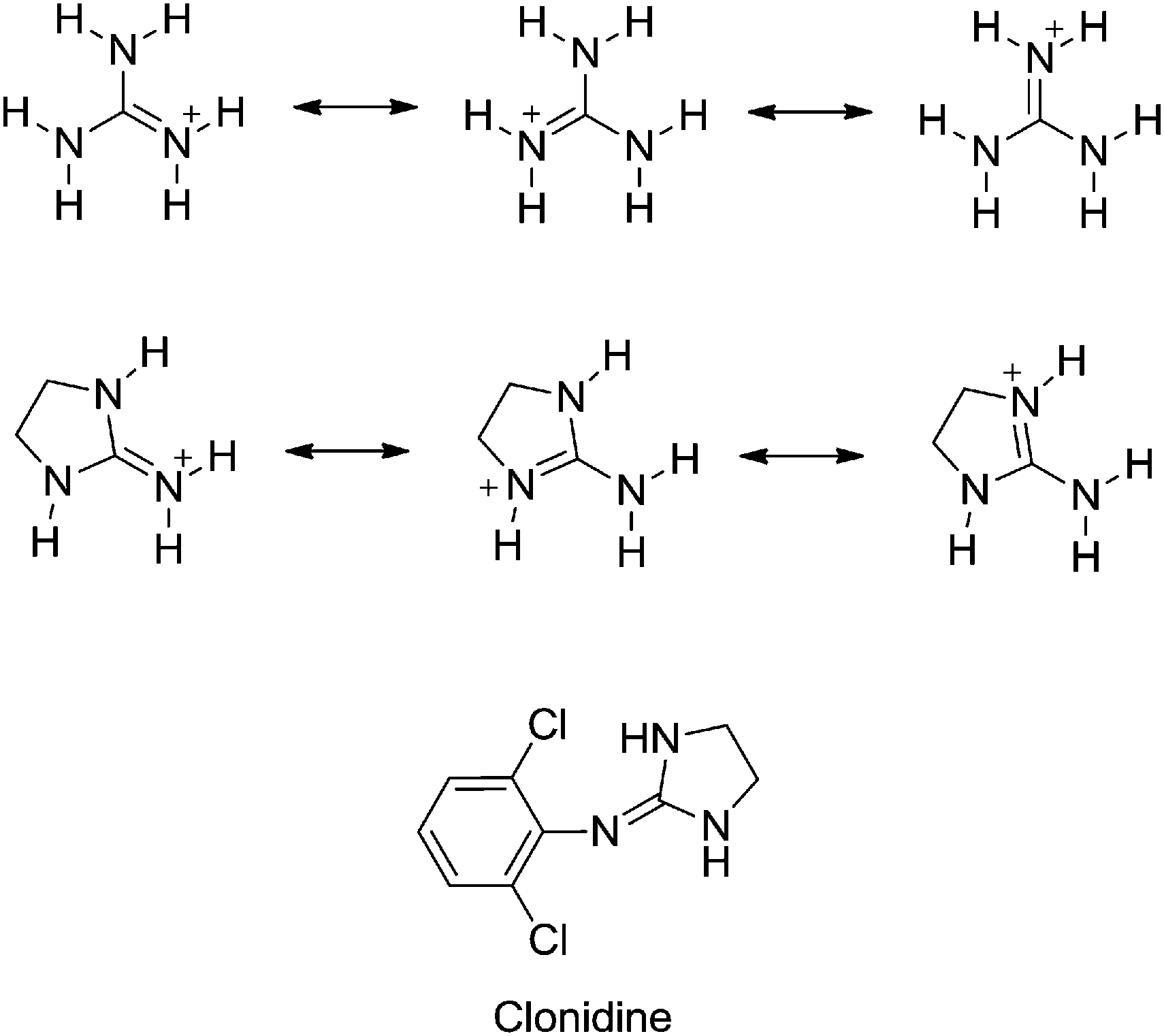


Substituent Effects On The Basicity P K A Of Aryl Guanidines And 2 Arylimino Imidazolidines Correlations Of Ph Metric And Uv Metric Values With P New Journal Of Chemistry Rsc Publishing Doi 10 1039 C7nje



Recent Advances In Guanidine Based Organocatalysts In Stereoselective Organic Transformation Reactions Intechopen
Guanidine, a nitrogen organic base, has been widely used in the synthesis of heterocyclic compounds incorporating at least two nitrogen atoms This moiety is present in the sidechain ofHydroxyl group in cat6 is an important secondary hydrogen bond donor, without this functional group, no reaction is observed Scheme 12 The cyclic guanidine ()ChibaG used as a chiral organocatalyst 12 Previous Strategies and Limitations for the Cyclization of GuanidinesAt present, coal is the main energy in our country, but a large number of coalfired has serious pollution Traditional coalfired, coalfired boilers and their power generation technologies all adopt the form of "burning coal by fire" The total energy efficiency and coal electricity conversion rate are low, pollution is serious, water consumption is large, and the cost of desulfurization
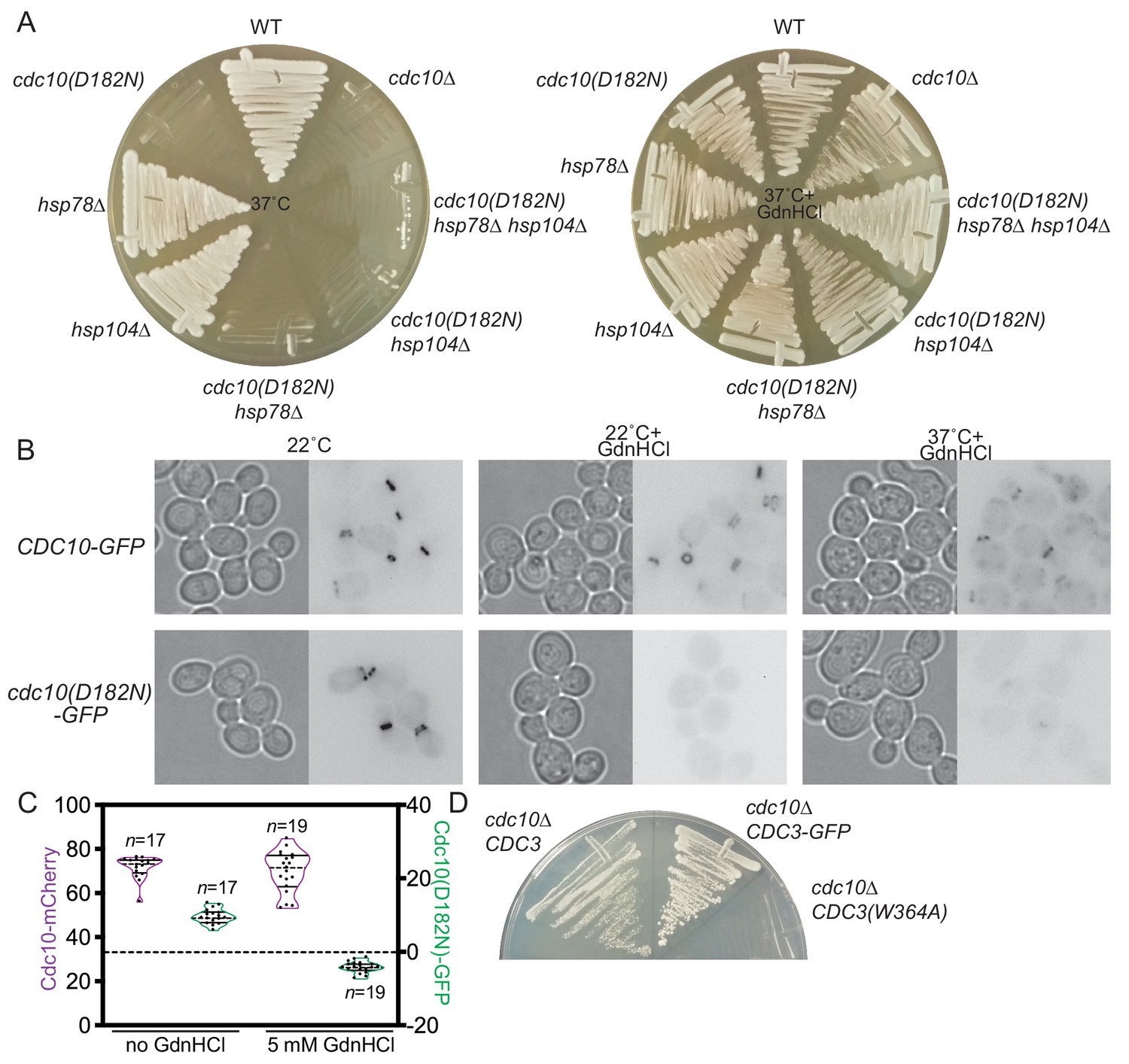


Guanidine Hydrochloride Reactivates An Ancient Septin Hetero Oligomer Assembly Pathway In Budding Yeast Elife



Guanidinium Salt An Overview Sciencedirect Topics
/11/15 · Guanidine is one of the most versatile functional groups in chemistry;The carbon nanotubes having guanidine groups, which are manufactured by the method of the current invention, are hydrogen bonded with the solvent molecule capable of reacting with the guanidine group to form the hydrogen bond, and thus, are uniformly dispersed in the solvent Further, by using the properties of the guanidine group capable of being selectively combined with crown ether, the carbon nanotubes having guanidineThe present study aimed to assess the effects of repeated exposure to lowdose Cd in a mouse model of polyhexamethylene guanidine (PHMG)induced lung fibrosis Mice were grouped into the following groups vehicle control (VC), PHMG, cadmium chloride (CdCl 2 ), and PHMG CdCl 2



Designed Guanidinium Rich Amphipathic Oligocarbonate Molecular Transporters Complex Deliver And Release Sirna In Cells Pnas



Guanidine Thiocyanate
· Guanidinium groups present in peptides and dendritic polymers induce their efficient transport through liposomal and cell membranes Transmembrane crossing of these polymers is affected by their structural features and is critically dependent on the number of guanidinium groups present Furthermore, the interaction of the guanidinium groups withThe guanidine moiety is present in many natural products where its catalytic activity has been established 34 Representative examples of these organocatalysts are shown in Figure 1 They include 7methyl1,5,7triazabicyclo440dec5ene (MTBD), DBU, and the aforementioned TBD In terms of basicity, TBD is more basic than MTBD and DBU pThe only hydrophilic group present in chlorpheniramine is the tertiary amine Question #3 Functional groups in Binding Interaction possible Chlorpheniramine with target of drug action Aromatic hydrocarbon Hydrophobic stacking Halogenated aromatic hydrocarbon Hydrophobic stacking Alkane Hydrophobic van der Waal Tertiary amine Hbonding Dipoledipole Iondipole



Amino Acid Amino Acid Amino Acid Proteins Many Peptide Linkages Pdf Free Download



Welcome To Chem Zipper Com Basicity Of Guanidine
Reiss et al present the 27 Å crystal structure of the guanidineI riboswitch aptamer, a bacterial mRNA element that specifically recognizes guanidinium to control downstream gene expression The structure reveals how the riboswitch specifically recognizes guanidinium while selecting against urea and larger metabolites containing guanidino moietiesThere are no $\mathbf{sp^3}$ nitrogens in guanidine Guanidine is isolobal to urea, where the carbonyl oxygen has been replaced by an imine $\ce{NH}$ However, in principle it is still the same flat, resonancestabilised molecule The main difference is that there is no 'preferred' site for the double bond — it could point towards any of the three nitrogens in theory;Guanidine, also called carbamidine, is a strongly alkaline and watersoluble compound that plays a key role in numerous biological activities The guanidine group de nes chemical and physicochemical properties of many compounds of medical interest1 Trimethoprim2 1, sulfadiazine3 2, and Gleevec (imatinib mesilate)4 3 are examples of pharmaceutically important guanidine
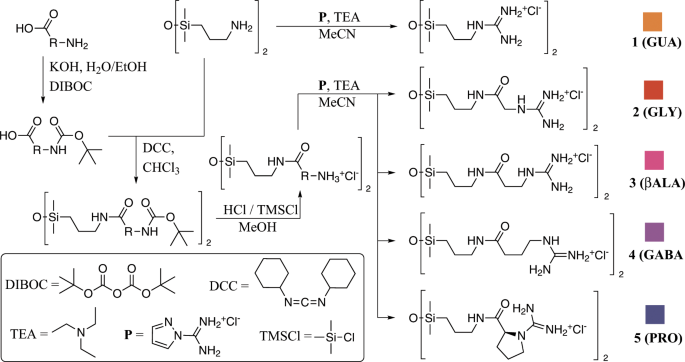


Exploring Ion Ion Preferences Through Structure Property Correlations Amino Acid Derived Bis Guanidinium Disiloxane Salts Scientific Reports
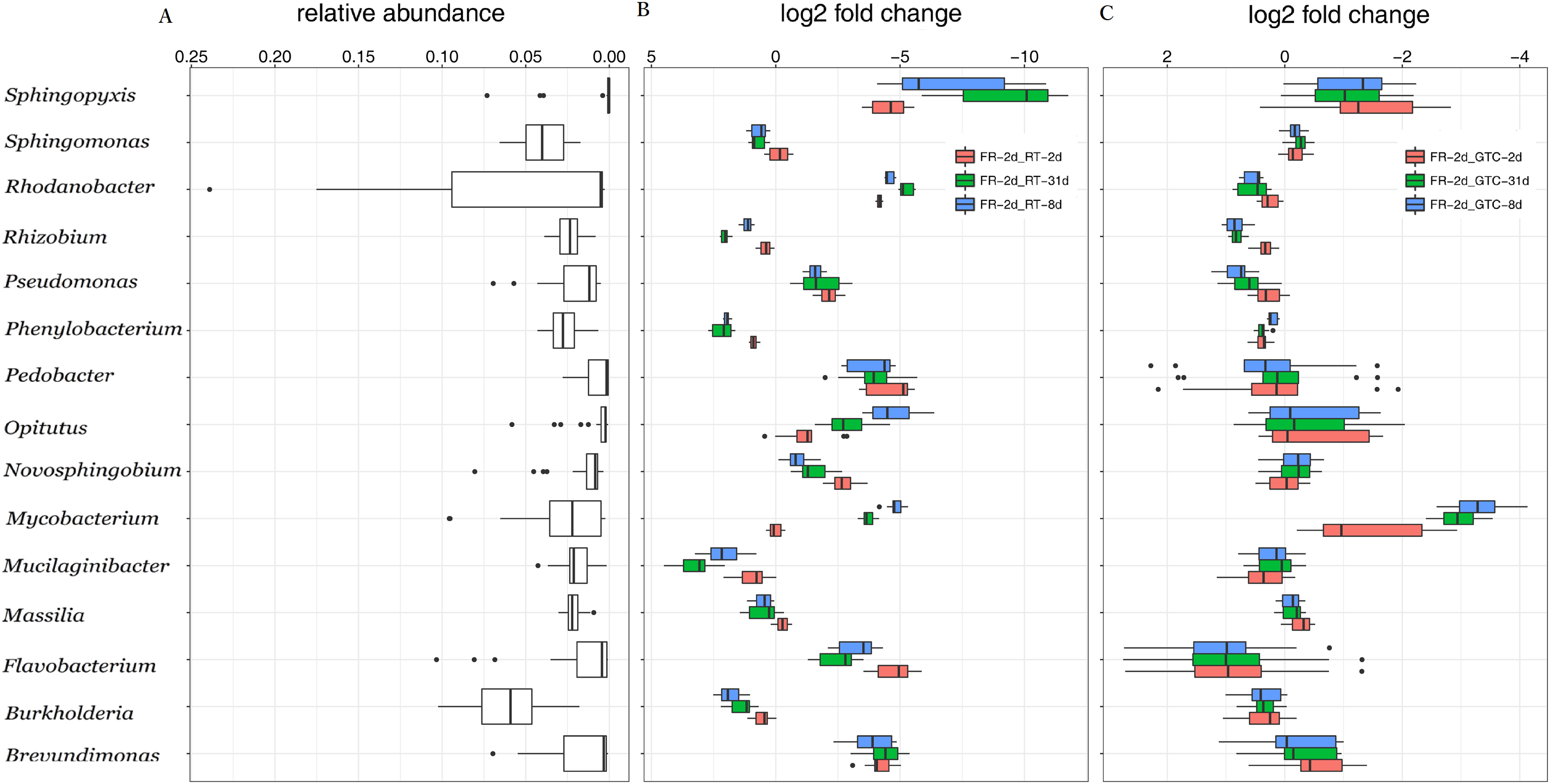


Guanidine Thiocyanate Solution Facilitates Sample Collection For Plant Rhizosphere Microbiome Analysis Peerj
An operationally simple and rapid coppercatalyzed threecomponent synthesis of trisubstituted N aryl guanidines involving cyanamides, arylboronic acids, and amines is performed in the presence of K 2 CO 3, a catalytic amount of CuCl 2 ·2H 2 O, bipyridine, and oxygen (1 atm) J Li, L Neuville, Org Lett, 13 , 15,'However, experience with guanidine and biguanides prompted the development of metformin' 'When guanidine hydrochloride is present these complexes dissociate into smaller units' 'Galega officinalis is rich in guanidine, the hypoglycemic component' 'RNA extraction was performed by the guanidine hydrochloride method'Compounds containing this system have found application in a diversity of biological activities, and in this chapter, the advances in the field of the synthesis of guanidines are presented First, the preparation of acyclic guanidines involving the reaction of an amine with an activated guanidine



Epb1 Methods For The Synthesis Of Polycyclic Guanidine Compounds Google Patents
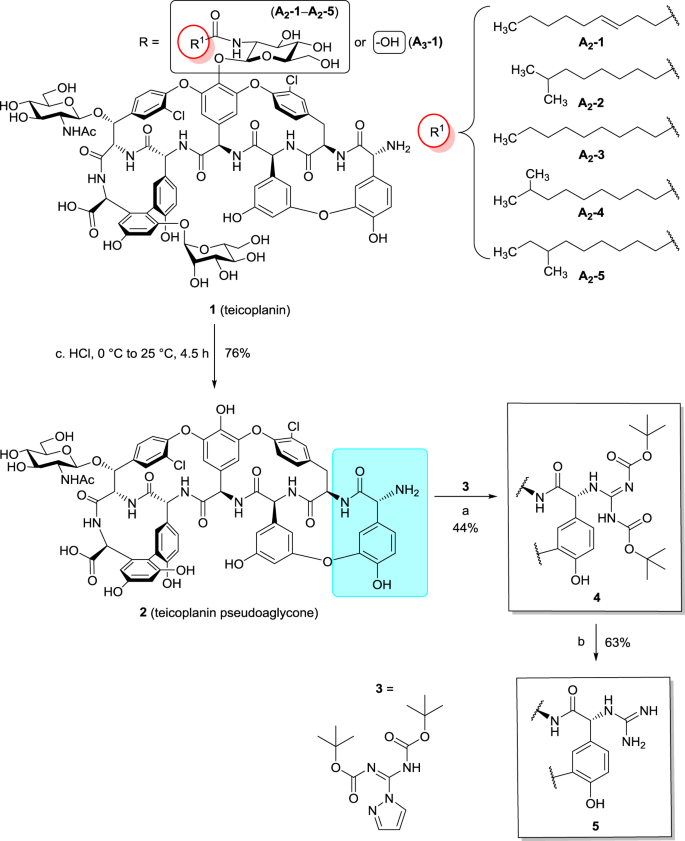


N Terminal Guanidine Derivatives Of Teicoplanin Antibiotics Strongly Active Against Glycopeptide Resistant Enterococcus Faecium The Journal Of Antibiotics
The guanidine group has attracted considerable attention since it is found in a wide array of natural and synthetic biologically active compounds 1,2,3 The guanidine groups are categorized as organosuperbases whose basicity is magnified because of the resonance stabilization of the corresponding conjugated acidsIndépendamment l'hydrogène, un halogène, un groupe amine, alkyle, hydroxy, alcoxy C16 ou sulfonylurée, Y, Q et Z pouvant être identiques ou différents, où le groupe alkylaryle est un groupe ayant un cycle aromatique unique à 6 atomes et le groupe alkyle a 1 à atomes de carbone, à la condition que dans le formule (I), lorsque X représente O, Y et Z ne représentent pas tous deux



1 2 Methyl 5 Nitrophenyl Guanidine Nitrate 08 7 Tci Shanghai Development Co Ltd



Botulism And Guanidine Nejm



The Chemical Structures Of L Arginine L Arg And L Arginine Methyl Download Scientific Diagram



Chemical Structure Of Three Amino Acids With Guanidinium Group Download Scientific Diagram



Protein Stiffening And Entropic Stabilization In The Subdenaturing Limit Of Guanidine Hydrochloride Biophysical Journal
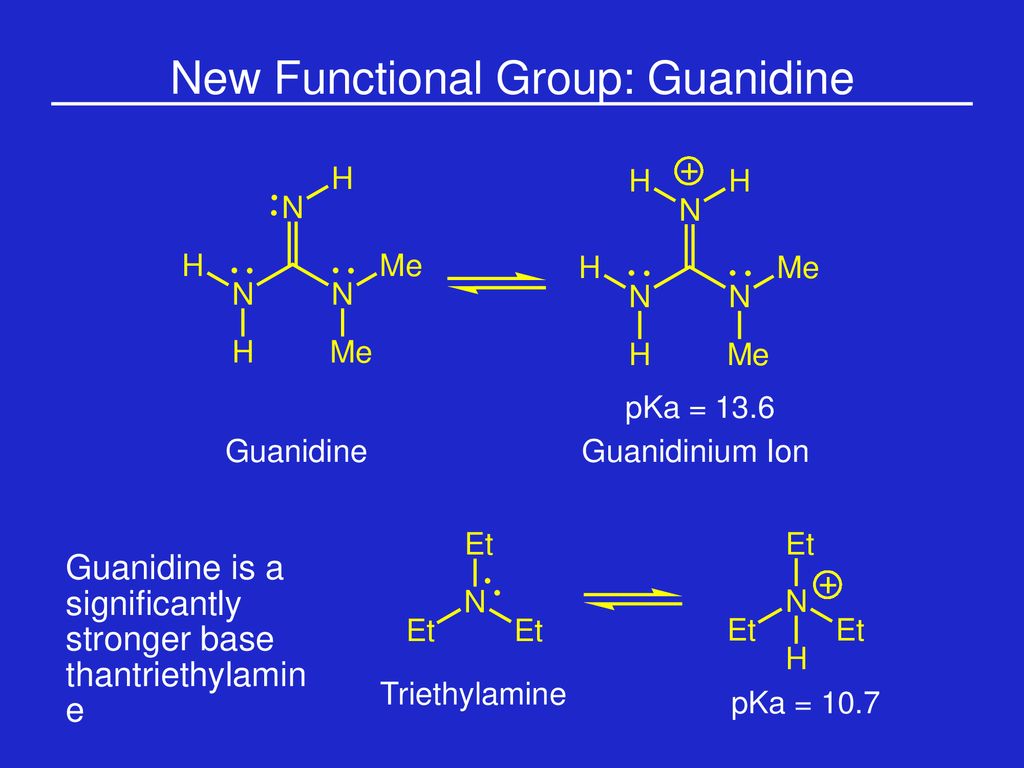


Week 1 Amino Acids Prof Sbw Ppt Download


Which Is The Most Basic Nitrogen In Guanidine Quora



Kra Methods For The Synthesis Of Polycyclic Guanidine Compounds Google Patents



Guanidine Synthesis Use Of Amidines As Guanylating Agents Baeten 16 Advanced Synthesis Amp Catalysis Wiley Online Library


Which Is The Most Basic Nitrogen In Guanidine Quora
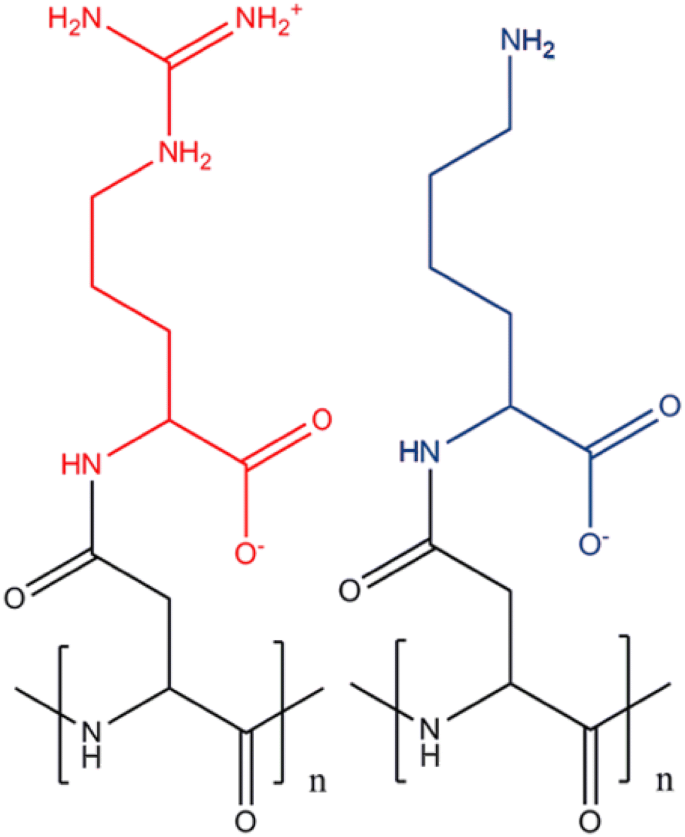


In Vitro Modification Of Bacterial Cyanophycin And Cyanophycin Dipeptides Using Chemical Agents Towards Novel Variants Of The Biopolymer Springerlink
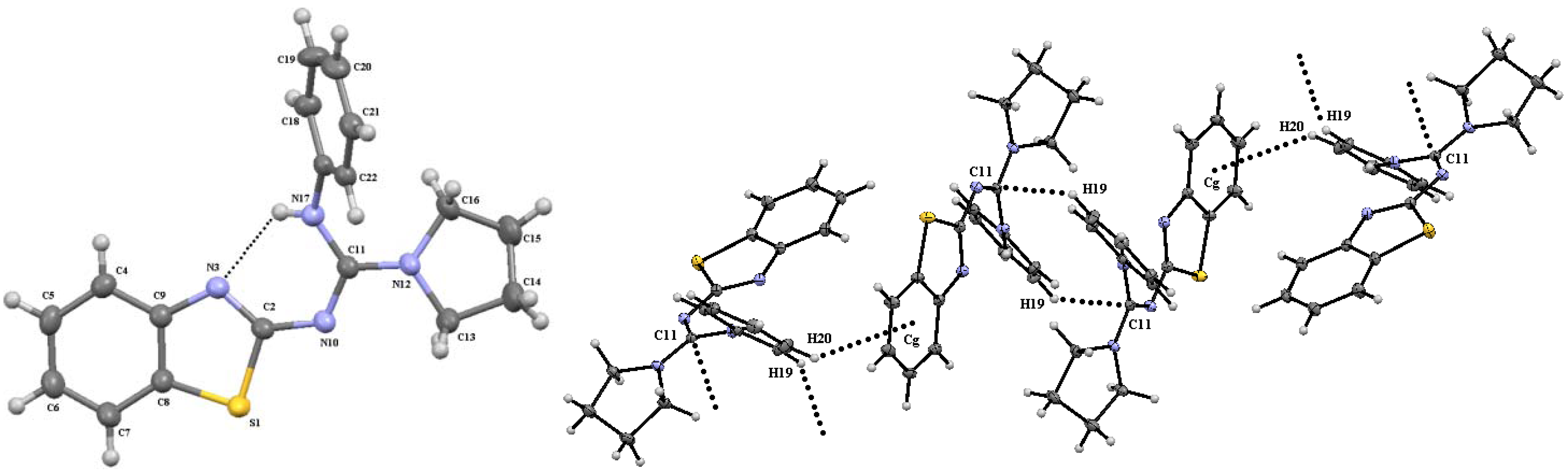


Molecules Free Full Text A Synthetic Method To Access Symmetric And Non Symmetric 2 N N Disubstituted Guanidinebenzothiazoles Html



Biochemical Validation Of A Fourth Guanidine Riboswitch Class In Bacteria Biorxiv


Solved Question 1 Which Of The Following Is An Accurat Chegg Com



Guanidine And The Guanidino Group Present In Arginine Are Two Of The Strongest Organic Bases Known Account For Their Basicity Homework Help And Answers Slader
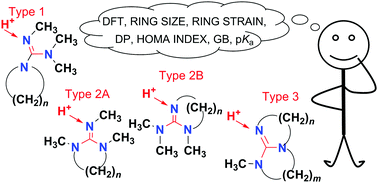


Gas Phase Basicity Of Cyclic Guanidine Derivatives A Dft Study New Journal Of Chemistry Rsc Publishing



Unusual Oxidative Chemistry Ofn W Hydroxyarginine And N Hydroxyguanidine Catalyzed At An Engineered Cavity In A Heme Peroxidase Journal Of Biological Chemistry


Csiro Publishing Australian Journal Of Chemistry



Guanidine Thiocyanate Santa Cruz Biotechnology


Chemical Structure Of Three Amino Acids With Guanidinium Group Download Scientific Diagram
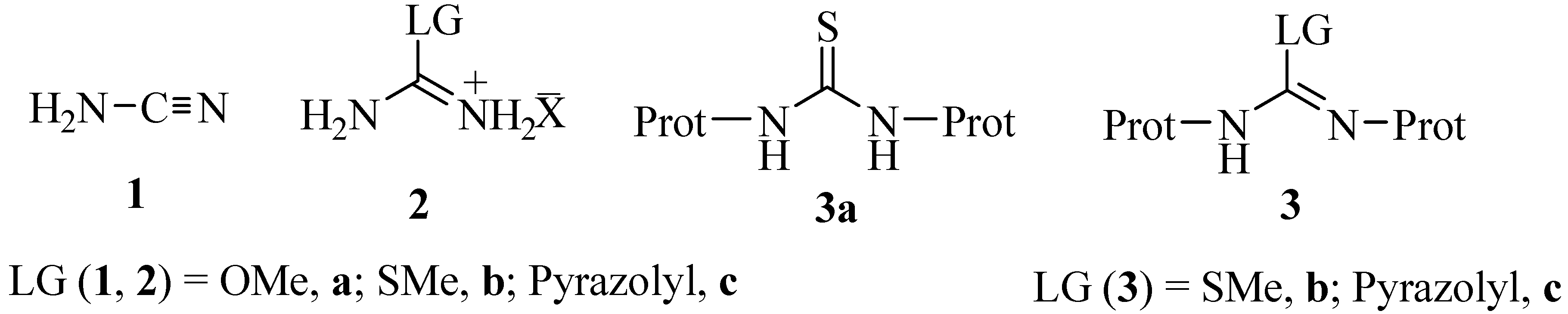


Molecules Free Full Text A Synthetic Method To Access Symmetric And Non Symmetric 2 N N Disubstituted Guanidinebenzothiazoles Html


Antibacterial Chemical And Physical Properties Of Sealants With Polyhexamethylene Guanidine Hydrochloride
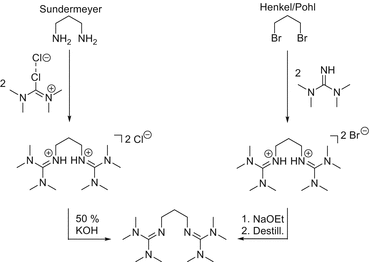


Guanidine Metal Complexes For Bioinorganic Chemistry And Polymerisation Catalysis Springerlink



Guanidine And Guanidinium Cation In The Excited State Theoretical Investigation The Journal Of Chemical Physics Vol 141 No 7



Mercaptoethylguanidine And Guanidine Inhibitors Of Nitric Oxide Synthase React With Peroxynitrite And Protect Against Peroxynitrite Induced Oxidative Damage Journal Of Biological Chemistry
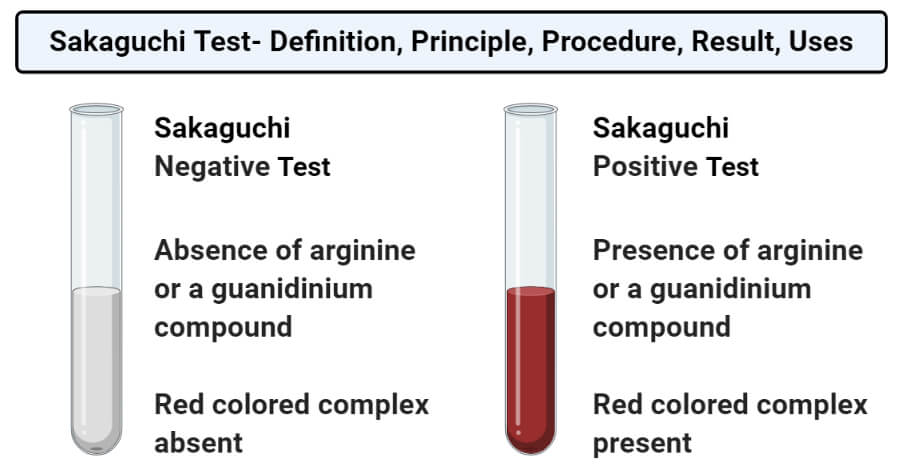


Sakaguchi Test Definition Principle Procedure Result Uses



Iucr Arginine Off Kilter Guanidinium Is Not As Planar As Restraints Denote



Guanidine Thiocyanate 99 Acros Organics 1kg Plastic Bottle Guanidine Thiocyanate 99 Acros Organics Fisher Scientific



Antimicrobial Drugs Bearing Guanidine Moieties A Review Sciencedirect



Guanidinium Group A Versatile Moiety Inducing Transport And Multicompartmentalization In Complementary Membranes Sciencedirect



Welcome To Chem Zipper Com Basicity Of Guanidine



Protonation Of Guanidine Chemistry Stack Exchange



Concise Synthesis Of Guanidine Containing Heterocycles Using The Biginelli Reaction Abstract Europe Pmc



Oneclass What Is The Chemical Structure Of Tetrodotoxin And What Functional Groups Are Present In It
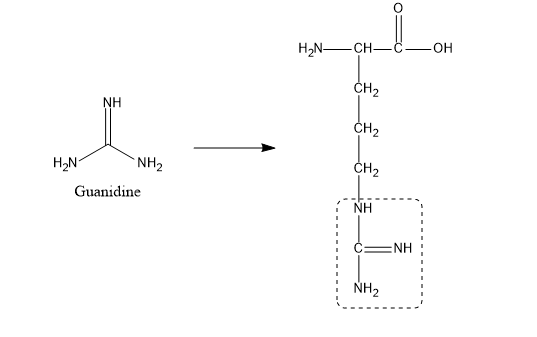


Guanidine And The Guanidino Group Present In Arginine Are Two Of The Strongest Organic Bases Known Account For Their Basicity Homework Help And Answers Slader


Disiloxanes And Functionalized Silica Gels One Route Two Complementary Outcomes Guanidinium And Pyridinium Ion Exchangers
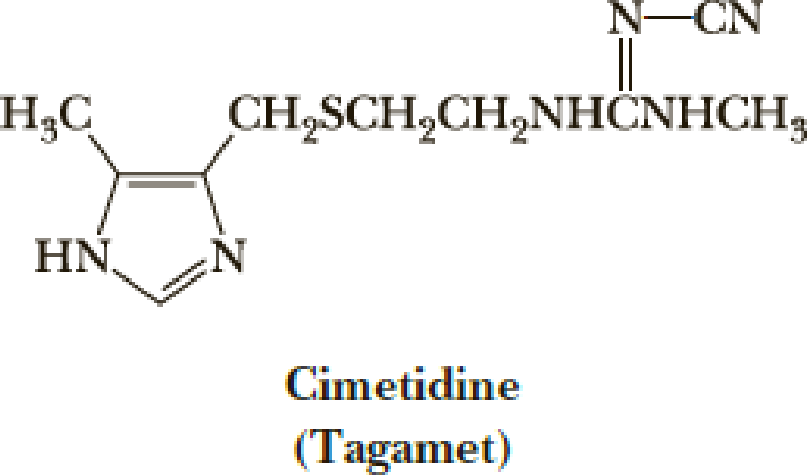


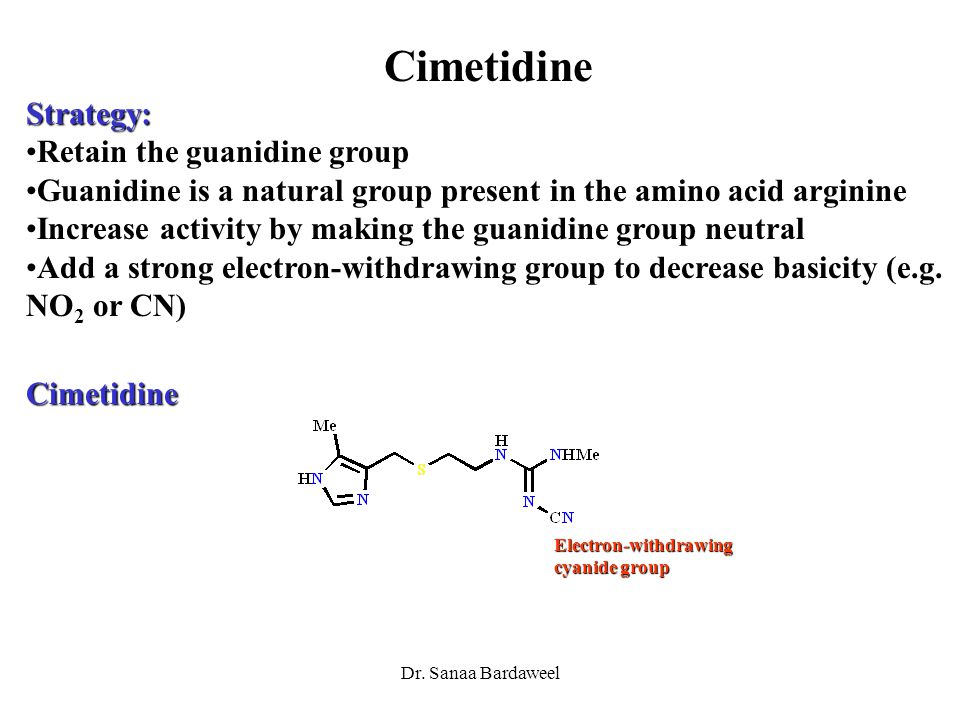
A Chemically Modified Guanidino Group Is Present In Cimetidine amet A Widely Prescribed Drug For The Control Of Gastric Acidity And Peptic Ulcers Cimetidine Reduces Gastric Acid Secretion By Inhibiting The Interaction



New Guanidine Borane Adducts An Experimental And Theoretical Approach Sciencedirect


Guanidine Ch5n3 Pubchem



Synthesis Of Guanidinium Modified Hyaluronic Acid Hydrogel Varghese 10 Macromolecular Rapid Communications Wiley Online Library



Pdf Guanidine Group Definition And Pharmaceutical Applications



Guanidine Derived Antimalarials And Their Drug Partners Used In Download Scientific Diagram



Urea But Not Guanidinium Destabilizes Proteins By Forming Hydrogen Bonds To The Peptide Group Pnas



Derivatives Of The Triaminoguanidinium Ion 6 Aminal Forming Reactions With Aldehydes And Ketones
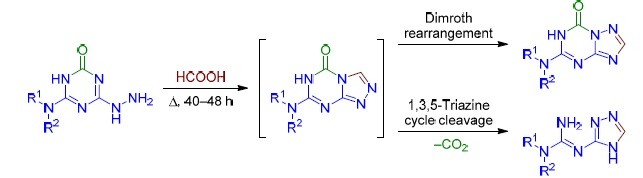


An Unexpected Direction Of The Reaction Of Hydrazino 1 3 5 Triazines With Formic Acid Synthesis Of 4 H 1 2 4 Triazol 3 Yl Guanidines Springerlink
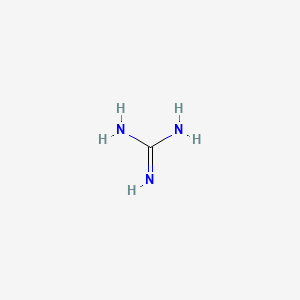


Guanidine Ch5n3 Pubchem



Hydrogen Ion Titration Curve Of Lysozyme In 6 M Guanidine Hydrochloride Pdf Document



Guanidinium Chloride Wikipedia



Guanidine Wikipedia



Guanidino Group An Overview Sciencedirect Topics



Guanidine And Guanidinium Cation In The Excited State Theoretical Investigation The Journal Of Chemical Physics Vol 141 No 7



Epb1 Methods For The Synthesis Of Polycyclic Guanidine Compounds Google Patents


Sakaguchi Test Principle Reaction Reagents Procedure And Result Interpretation Online Biochemistry Notes



Assisting The Reactivation Of Guanidine Hydrochloride Denatured Aminoacylase By Hydroxypropyl Cyclodextrins Biophysical Journal
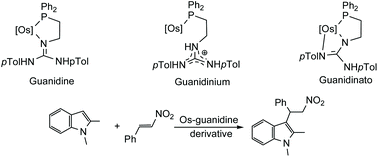


Half Sandwich Complexes Of Osmium Containing Guanidine Derived Ligands Dalton Transactions Rsc Publishing
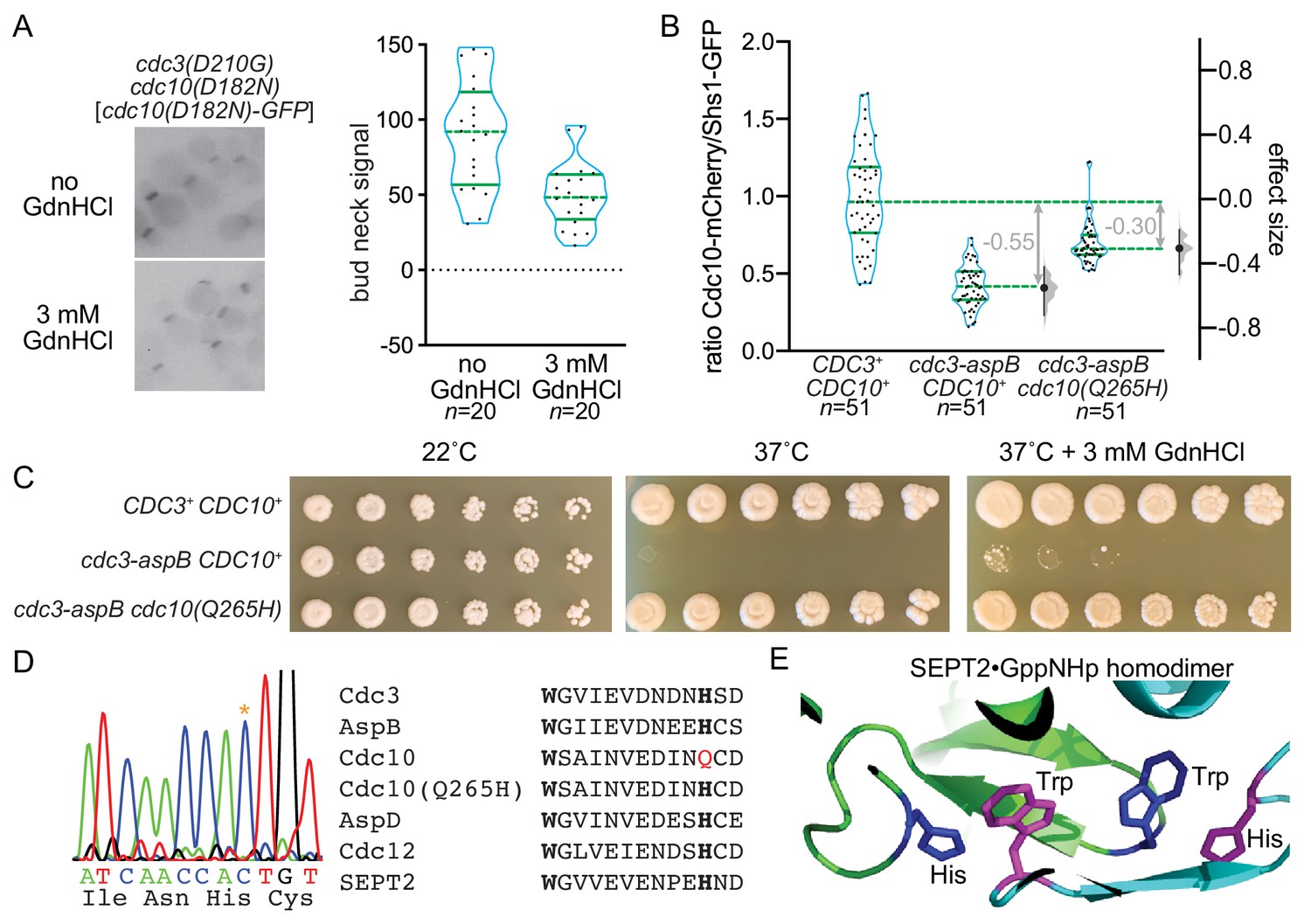


Guanidine Hydrochloride Reactivates An Ancient Septin Hetero Oligomer Assembly Pathway In Budding Yeast Elife



Dendritic Guanidines As Efficient Analogues Of Cell Penetrating Peptides Abstract Europe Pmc
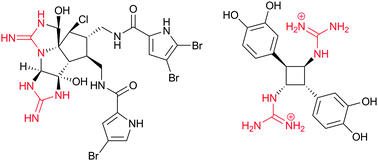


The Chemistry And Biology Of Organic Guanidine Derivatives Natural Product Reports Rsc Publishing



Guanidine An Overview Sciencedirect Topics
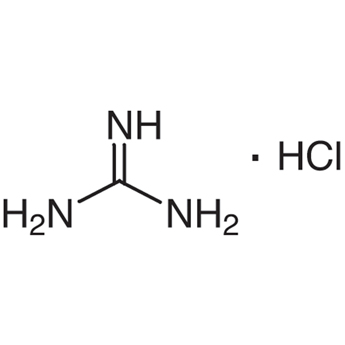


Guanidine Hydrochloride 50 01 1 Tokyo Chemical Industry Co Ltd Apac
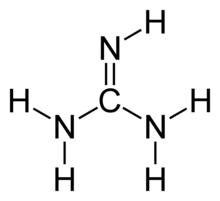


Guanidine Wikiwand



Ep A1 Novel Guanidine Derivatives As Inhibitors Of Cell Adhesion The Lens Free Open Patent And Scholarly Search



Biochemical Validation Of A Fourth Guanidine Riboswitch Class In Bacteria Biorxiv
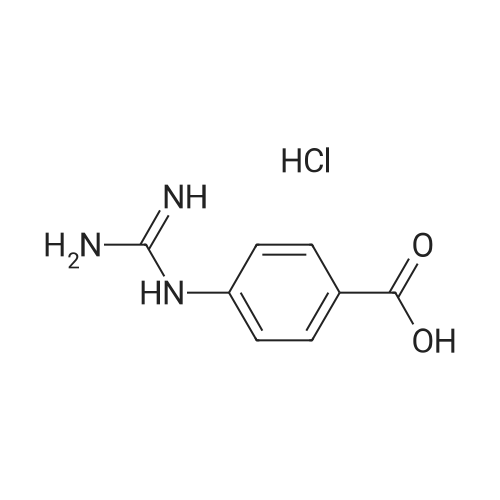


Vhffxctv6y1akm
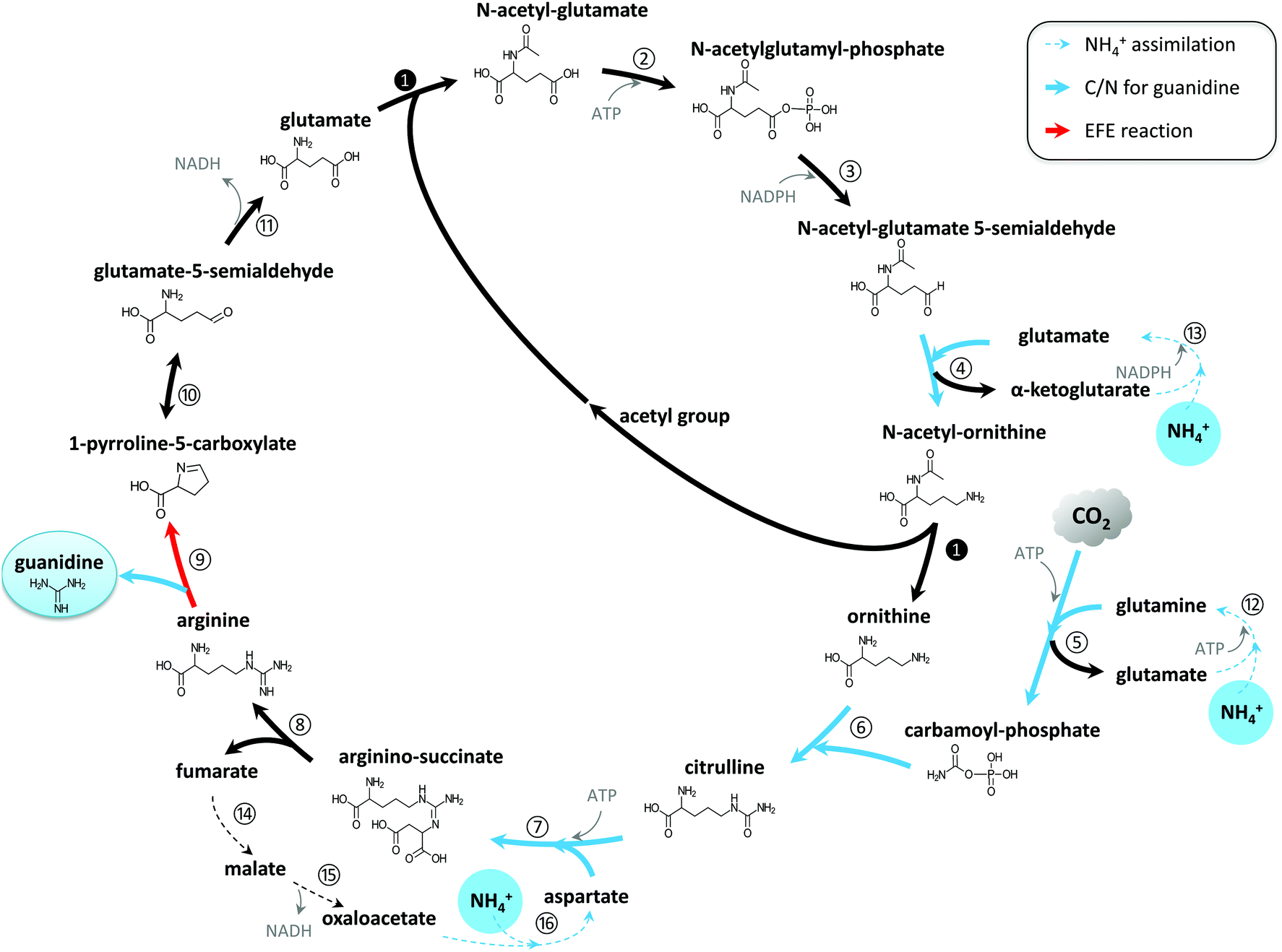


Photosynthetic Production Of The Nitrogen Rich Compound Guanidine Green Chemistry Rsc Publishing Doi 10 1039 C9gcc



Guanidine Formula Uses Facts Britannica



Guanidine Carbonate 593 85 1 Tci America



Antimicrobial Drugs Bearing Guanidine Moieties A Review Sciencedirect
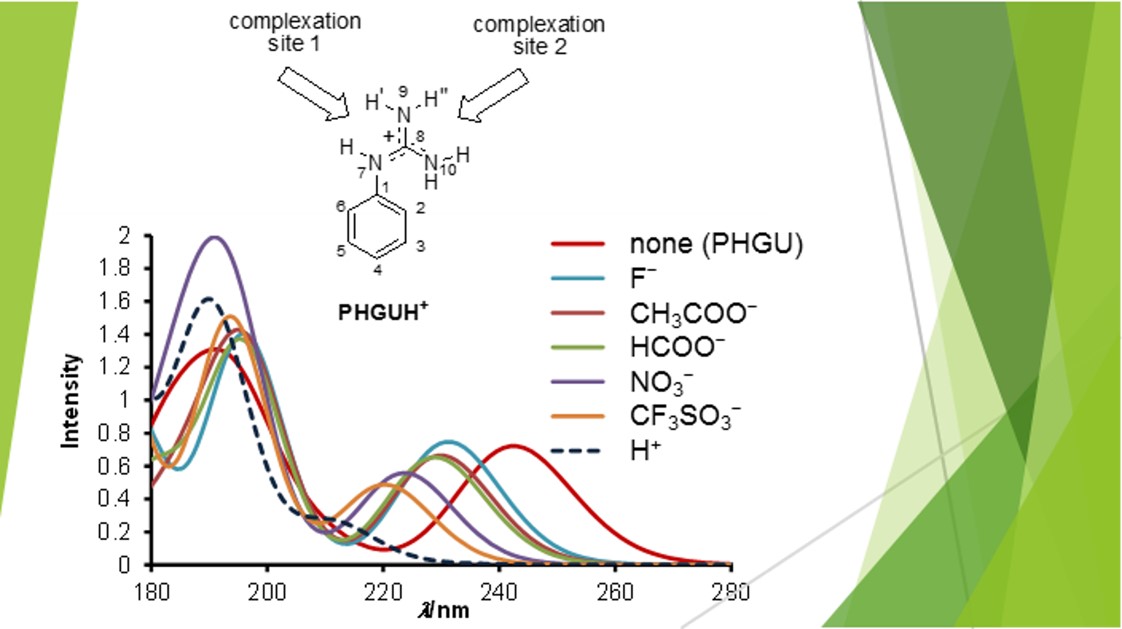


Guanidinium A New Analytical Tool To Detect Anions Light And Molecules



Kra Methods For The Synthesis Of Polycyclic Guanidine Compounds Google Patents



No comments:
Post a Comment